Glecaprevir
Appearance: White to Off-White powder
Assay: 98%
Brand: YIHUI
Packing: 1g;10g;100g
Supply Ability: 2000g per monthShelf Life: Two years
Application: lab research
Payment: T/T, LC or DA
Sample: available
Delivery Time: Ready Stock in Local Warehouse,1-3 days
Prompt and Secure Shipment
Origin: China
Shipping: DHL,FedEx,TNT,EMS,By Sea,By Air
Certifications: ISO9001, ISO22000, HACCP, HALAL, KOSHER
Can't sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
What is Glecaprevir?
Glecaprevir is an antiviral drug that is utilized to treat hepatitis C infection (HCV) disease. Because it is a protease inhibitor, it prevents the HCV protease, an enzyme necessary for the virus to replicate, from working. Glecaprevir is regularly utilized in mix with other antiviral meds, for example, sofosbuvir, to accomplish high paces of fix.
HCV is a blood-borne infection that can cause liver harm, cirrhosis, and liver malignant growth. The infection recreates by capturing the host cell's hardware and utilizing it to deliver new duplicates of the infection. Glecaprevir works by restricting to the HCV protease and keeping it from severing a key protein that is essential for viral replication. The virus cannot replicate and spread as a result of this.
It is ordinarily utilized in blend with other antiviral prescriptions to treat HCV contamination. The combination of glecaprevir and sofosbuvir, sold under the brand name Epclusa, is the most common. Epclusa is a once-everyday pill that is taken for a long time. It can likewise be utilized in blend with other antiviralmedications, such as pibrentasvir and voxilaprevir. These combinations are typically used to treat people with more advanced HCV infection or people who have failed previous treatment regimens.
Glecaprevir-based regimens have been shown to be highly effective in treating HCV infection. In clinical trials, Epclusa achieved sustained virologic response (SVR) rates of over 95% in people with chronic HCV infection. SVR is defined as having no detectable virus in the blood 12 weeks after completing treatment.
Glecaprevir is generally well-tolerated. The most common side effects are fatigue, headache, and nausea. These side effects are typically mild and go away within a few weeks.
Basic Information
Product Name: Glecaprevir
CAS: 1365970-03-1
MF:C38H46F4N6O9S
MW:838.87
MDL No.:MFCD30533436
form Solid
color White to Off-White
Purity: 98%+
Package: 1g; 5g; 25g
Used: Lab research
Stability: Stable
Structural formula:
Specification Parameter:
Item | Specification | Results |
Appearance | Gray powder | Complies |
Purity(HPLC) | ≥98% | 98.3% |
Conclusion | It complies with the requirements | |
Chemical Composition:
Glecaprevir's chemical composition is meticulously crafted, ensuring its efficacy and safety. The primary components include carbon (C), hydrogen (H), nitrogen (N), and oxygen (O). The precise arrangement of these elements contributes to Glecaprevir's remarkable properties and pharmaceutical effectiveness.
Compound piece alludes to the basic and sub-atomic cosmetics of a substance. It tends to be communicated as far as the components that make up the substance, the particles that are available, or the synthetic recipe of the substance.
The natural sythesis of a substance alludes to the components that make up the substance and the general measures of every component. For instance, the basic piece of water is hydrogen and oxygen, with two hydrogen iotas for each oxygen particle. The basic piece of a substance can be resolved utilizing different logical methods, like natural examination, spectroscopy, and mass spectrometry.
The sub-atomic creation of a substance alludes to the particles that are available in the substance and the overall measures of every particle. Water, for instance, has a molecular composition of H2O, which indicates that each molecule contains one oxygen atom and two hydrogen atoms. The sub-atomic creation of a substance can be resolved utilizing various scientific strategies, like chromatography, spectroscopy, and mass spectrometry.
Effects and Functions:
Glecaprevir is renowned for its potent antiviral properties, particularly in combating hepatitis C virus (HCV) infections. It inhibits the HCV NS3/4A protease, a crucial enzyme for viral replication, thereby interrupting the life cycle of the virus. The effectiveness of Glecaprevir extends to a broad spectrum of HCV genotypes, making it a versatile and reliable solution in the field of antiviral therapy.
Hepatitis C virus (HCV) infection can be treated with the antiviral drug glecaprevir. It is a protease inhibitor, which means it prevents the HCV protease enzyme from working. This catalyst is fundamental for the replication of the HCV infection. By impeding the action of the protease chemical, glecaprevir keeps the infection from recreating and spreading.
It is normally utilized in blend with other antiviral medications, for example, sofosbuvir, to treat HCV disease. This blend treatment is exceptionally viable at restoring HCV disease, with fix paces of more than 95%.The most widely recognized results of glecaprevir incorporate weariness, migraine, and queasiness. These secondary effects are normally gentle and disappear following half a month of treatment.Glecaprevir's capability is to hinder the movement of the HCV protease catalyst. This keeps the infection from recreating and spreading, which can prompt a solution for HCV contamination.It is a powerful and specific inhibitor of the HCV protease catalyst. It is capable of inhibiting the enzyme's activity at extremely low concentrations and has a high binding affinity for the enzyme. This makes it an extremely viable medication for treating HCV contamination.
Glecaprevir is a protected and compelling medication for treating HCV contamination. It is regularly utilized in mix with other antiviral medications, for example, sofosbuvir, to accomplish high fix rates. Glecaprevir can restrain the action of the HCV protease chemical, which keeps the infection from recreating and spreading.
Synthesis Process:
The synthesis process of Glecaprevir involves a series of precise and controlled steps to ensure the purity and quality of the final product. Starting with carefully selected raw materials, the synthesis proceeds through intricate chemical reactions, guided by stringent quality control measures.
The first step is to synthesize the pyrrolidine ring, which is a key structural component of glecaprevir. This is done by reacting a ketone with an amine in the presence of a reducing agent.
The next step is to alkylate the pyrrolidine ring with an alkyl halide. This reaction is typically carried out in the presence of a base.
The third step is to form the imidazole ring, which is another key structural component of glecaprevir. This is done by reacting the alkylated pyrrolidine ring with an aldehyde in the presence of an acid catalyst.
The fourth step is to couple the imidazole and pyrrolidine rings together. This is done by reacting the imidazole ring with an activated form of the pyrrolidine ring.
The fifth step is to introduce the trifluoromethyl group onto the glecaprevir molecule. This is done by reacting glecaprevir with a trifluoromethylating agent.
The final step is to purify glecaprevir to remove any impurities. This may involve recrystallization or chromatography.
The synthesis of glecaprevir is a complex and challenging process, but it is essential for the production of this important antiviral drug.
The synthesis begins with the assembly of key intermediate compounds, which are then further refined to produce the final Glecaprevir product. Yihui's state-of-the-art facilities and experienced chemists guarantee a reliable and reproducible synthesis process, meeting the highest industry standards.
Quality Standards:
Yihui upholds a commitment to excellence in manufacturing Glecaprevir, adhering to stringent quality standards. The production process is conducted in compliance with international regulations, and the finished product undergoes rigorous quality testing. Yihui proudly holds certifications for ISO, Kosher, Halal, and GMP (Good Manufacturing Practice), underscoring our dedication to delivering pharmaceutical products of the highest caliber.
The quality standards for glecaprevir include:
Identity: The drug substance must be glecaprevir and not another compound.
Purity: The drug substance must be free of impurities, such as starting materials, intermediates, and degradation products.
Potency: The drug substance must contain the correct amount of active ingredient.
Safety: The drug substance must be safe for use in patients. This includes testing for potential genotoxicity, carcinogenicity, and reproductive toxicity.
Efficacy: The drug substance must be effective in treating the intended disease or condition.
Appearance: The drug substance should be a white to off-white powder.
Solubility: The drug substance should be soluble in water and organic solvents.
Stability: The drug substance should be stable under a variety of conditions, including temperature, humidity, and light.
Makers of glecaprevir should follow Great Assembling Practices (GMP) to guarantee that the medication substance meets the expected quality guidelines as a whole. GMPs are a bunch of guidelines that oversee the assembling, testing, and dispersion of drug items
Application Fields in Various Industries:
Glecaprevir's multifaceted applications extend beyond the realm of pharmaceuticals. Its significance is recognized across diverse industries, including healthcare, research institutions, and pharmaceutical companies. The compound's versatility makes it a valuable tool in studying viral infections and developing innovative treatments.
It is used as an intermediate in the synthesis of other HCV drugs.
It is also used in the development of new HCV drugs and treatments.
It is used in research studies to investigate the mechanisms of HCV replication and to develop new treatments for HCV infection.
It may also be used in the following industries:Veterinary medicine: Glecaprevir may be used to treat HCV infection in animals.
Food and beverage industry: It may be used to prevent HCV contamination of food and beverages.
Environmental industry: It may be used to treat HCV-contaminated water and soil.
Packing & Shipping
 Packing:
Packing:
1kg/foil bag;5kg/carton;25kg/fiber drum; or packing as your request.
Customization:
l Customized logo
l Customized packaging
l Graphic customization
Shipping:
By Courier; By Air or By Sea, according to your demands
Payment Term

Why Choose Xi'an Yihui?
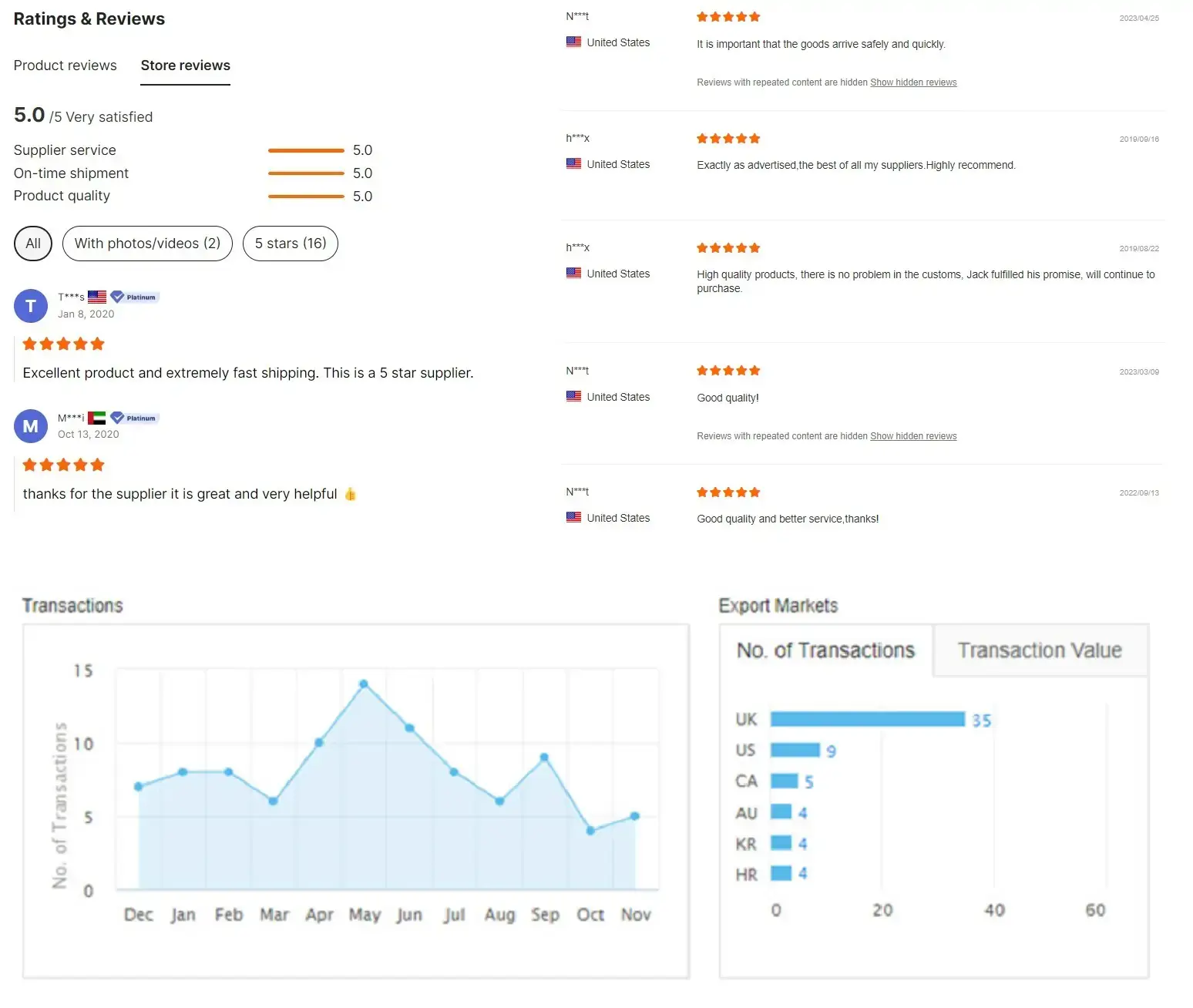
Customer feedback
Xi'an Yihui certificates

Welcome to Xi'an Yihui Factory

Our Advantage
Rich experience: we have 13 Years of professional experience;
Customers all over the world: sell to more than 100 countries;
Provide diversified products: the products have been applied to all major international brands in the fields of drugs, dietary supplements, cosmetics, animal nutrition and functional food.
Price advance: low MOQ with competitive price;
Quality certification: ISO; Halal; Kosher certified
After-sales service: Professional team 7*24 hours customer service.
Contact Us:
Yihui emerges as a beacon of trust in the pharmaceutical industry, consistently delivering high-quality Glecaprevir that aligns with international standards. Our ISO, Kosher, Halal, and GMP certifications underscore our commitment to excellence. For inquiries and purchases, please contact us at sales@yihuipharm.com.

.webp)

.webp)
.webp)




 MK-7.webp)

