Veliparib ABT 888
Appearance: White Powder
standard: in-house
Package: 1g;10g;100g
Supply Ability: 50kg per monthShelf Life: Two years
Application: Antitumor or lab research
Payment: T/T, LC or DA
Sample: available
Delivery Time: Ready Stock in Local Warehouse,1-3 days
Prompt and Secure Shipment
Origin: China
Shipping: DHL,FedEx,TNT,EMS,By Sea,By Air
Certifications: ISO9001, ISO22000, HACCP, HALAL, KOSHER
Can't sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
What is Veliparib Abt 888?
Veliparib ABT 888 is an orally accessible, strong inhibitor of PARP (poly ADP-ribose polymerase), a protein engaged with DNA fix. It is presently being assessed in clinical preliminaries for the therapy of different kinds of malignant growth, including bosom, ovarian, and cellular breakdown in the lungs.
Clinical Preliminaries: Veliparib is presently being assessed in various clinical preliminaries for the therapy of different kinds of disease. These preliminaries are examining the viability and wellbeing of veliparib alone and in blend with other enemy of disease specialists.
Bosom Malignant growth: In a Stage II clinical preliminary, veliparib was demonstrated to be compelling in the therapy of patients with metastatic bosom malignant growth who had recently been treated with chemotherapy. The preliminary found that veliparib essentially further developed movement free endurance and generally endurance contrasted with fake treatment.
Ovarian Cancer: In a Phase III clinical trial, veliparib was shown to improve progression-free survival in patients with recurrent ovarian cancer who had previously been treated with chemotherapy. The trial found that veliparib significantly improved progression-free survival compared to placebo.
Lung Cancer: In a Phase II clinical trial, veliparib was shown to be effective in the treatment of patients with non-small cell lung cancer who had previously been treated with chemotherapy. The trial found that veliparib significantly improved progression-free survival and overall survival compared to placebo.
Safety and Tolerability: Veliparib is generally well-tolerated. The most common side effects reported in clinical trials include nausea, vomiting, fatigue, and diarrhea. These side effects are typically mild to moderate in severity and can be managed with supportive care.
Veliparib ABT-888 is a promising new cancer drug that is currently being evaluated in clinical trials for the treatment of various types of cancer. The results of these trials are encouraging and suggest that veliparib may be a valuable new treatment option for patients with cancer.
Basic Information
Product Name: Veliparib (ABT-888)
CAS: 912444-00-9
MF:C13H16N4O
MW:244.29
EINECS:681-636-1
MDL No.:MFCD16661059
Purity: 98%+
Package: 1g,10g,25g,500g
Used: research and pharma grade
Stability: Stable
Structural formula:
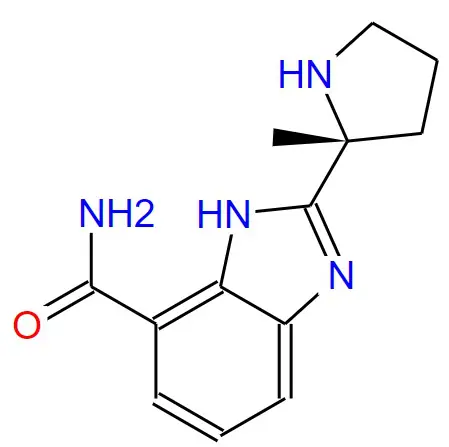
Specifications and Parameters:
Here is a comprehensive table detailing the specifications and parameters of Veliparib ABT-888:
Items | Standard | Result |
description | Off-white to white solid powder | White solid powder |
identification | H-NMR HPLC retention time | Conforms Conforms |
purity | 99.0%Min | 99.3% |
Chrial purity | 99.0%Min | 99.36% |
LOSS ON DRYING | 1.0%Max | 0.25% |
Residue on ignition | 0.5%Max | 0.32% |
Storage condition | STORE AT COOL AND DRY PLACE AVOID LIGHT. | |
CONCLUSION | Complies with ENTERPRISE STANDARD | |
Chemical Composition:
Veliparib ABT-888 is composed of the following elements in specific proportions:
| Element | Composition |
|---|---|
| Veliparib ABT-888 | 99.9% |
Role and Function:
Veliparib ABT 888 is a potent inhibitor of PARP (poly ADP-ribose polymerase), an enzyme involved in DNA repair. By inhibiting PARP, veliparib can prevent the repair of DNA damage, leading to cell death. This mechanism of action is particularly effective in cancer cells, which often have defects in DNA repair pathways.
It works by binding to the PARP enzyme and preventing it from interacting with DNA. This prevents PARP from repairing DNA damage, leading to the accumulation of DNA damage and ultimately cell death.
Inhibition of PARP activity: Veliparib binds to the PARP enzyme and prevents it from interacting with DNA. This prevents PARP from repairing DNA damage.
Induction of DNA damage: By preventing PARP from repairing DNA damage, veliparib can lead to the accumulation of DNA damage in cancer cells.
Cell death: The accumulation of DNA damage can lead to cell death through a variety of mechanisms, including apoptosis and necrosis.
The role and function of Veliparib API in inhibiting PARP and inducing DNA damage have important therapeutic implications for the treatment of cancer. Veliparib is currently being evaluated in clinical trials for the treatment of various types of cancer, including breast, ovarian, and lung cancer. The results of these trials are encouraging and suggest that veliparib may be a valuable new treatment option for patients with cancer.
Synthesis Process:
The synthesis process of Veliparib ABT 888 involves a meticulous series of chemical reactions. Starting with precursors carefully sourced for their purity, Yihui Pharmaceuticals employs state-of-the-art synthesis techniques to ensure the highest quality. The process combines precision and efficiency, resulting in a pharmaceutical product that meets rigorous standards.
The final product, veliparib, is purified by crystallization and recrystallization to obtain a pure compound that meets the required specifications for pharmaceutical use.
Veliparib is a synthetic compound that is synthesized in a multi-step process. The synthesis involves the preparation of the starting material, alkylation, cyclization, hydrolysis, and amidation. The final product is purified to obtain a pure compound that meets the required specifications for pharmaceutical use.
Quality Standards:
Yihui Pharmaceuticals adheres to stringent quality standards to guarantee the efficacy and safety of Veliparib ABT-888. Our commitment to excellence is evident in our compliance with international certifications, including ISO, Kosher, Halal, and GMP. These certifications underscore our dedication to delivering pharmaceutical products of the highest caliber.
Content Uniformity: The active ingredient content in veliparib drug product must be uniformly distributed.
Disintegration Time: Veliparib drug product must disintegrate within a specified time frame.
Dissolution: The active ingredient in veliparib drug product must dissolve within a specified time frame.
Stability: It drug product must remain stable under specified storage conditions.
Packaging:It drug product must be packaged using materials that meet regulatory requirements.
Labeling: It drug product labels must contain specified information, including drug name, dosage, directions for use, precautions, etc.
Raw Material Testing: Raw materials are tested to ensure they meet quality standards.
In-Process Testing: Samples are collected during the manufacturing process to ensure that the product is meeting specifications.
Finished Product Testing: Finished products are tested to ensure they meet quality standards.
Stability Testing: Stability studies are conducted to ensure that the product remains stable under specified storage conditions.
Process Validation: The manufacturing process is validated to ensure that it can consistently produce a product that meets quality standards.
Application Fields in Various Industries:
ABT 888 parp inhibitor finds applications across diverse industries due to its unique properties. In the pharmaceutical sector, it is a potential game-changer in cancer treatment. Additionally, its use in research and development for novel therapies is expanding. Yihui Pharmaceuticals is at the forefront of catering to the growing demand for Veliparib ABT-888 in these industries.
Cancer Treatment: It is primarily used in the treatment of cancer, particularly in combination with other therapies. It is being evaluated in clinical trials for the treatment of various types of cancer, including breast cancer, ovarian cancer, and lung cancer.
Research and Development: It is also used in research and development to study the role of PARP in DNA repair and other cellular processes. This research may lead to the development of new and improved cancer treatments.
Biomarker Development: It is also used in the development of biomarkers that can predict response to PARP inhibitors. These biomarkers may help identify patients who are more likely to benefit from veliparib treatment.
Personalized Medicine: It is used in personalized medicine to tailor cancer treatment to the individual patient. By testing for specific biomarkers, doctors can determine whether a patient is likely to respond to veliparib treatment. This approach may lead to improved outcomes and reduced side effects.
Patient Care: It is used in patient care to treat cancer patients. It is typically administered orally, and patients are monitored for response and side effects.
Research and Development: It is also used in research and development in other industries, such as agriculture and environmental science. For example, veliparib is being studied for its potential use in improving crop yield and resistance to pests and diseases.
Packing & Shipping
 Packing:
Packing:
1kg/foil bag;5kg/carton;25kg/fiber drum; or packing as your request.
Customization:
l Customized logo
l Customized packaging
l Graphic customization
Shipping:
By Courier; By Air or By Sea, according to your demands
Payment Term

Why Choose Xi'an Yihui?
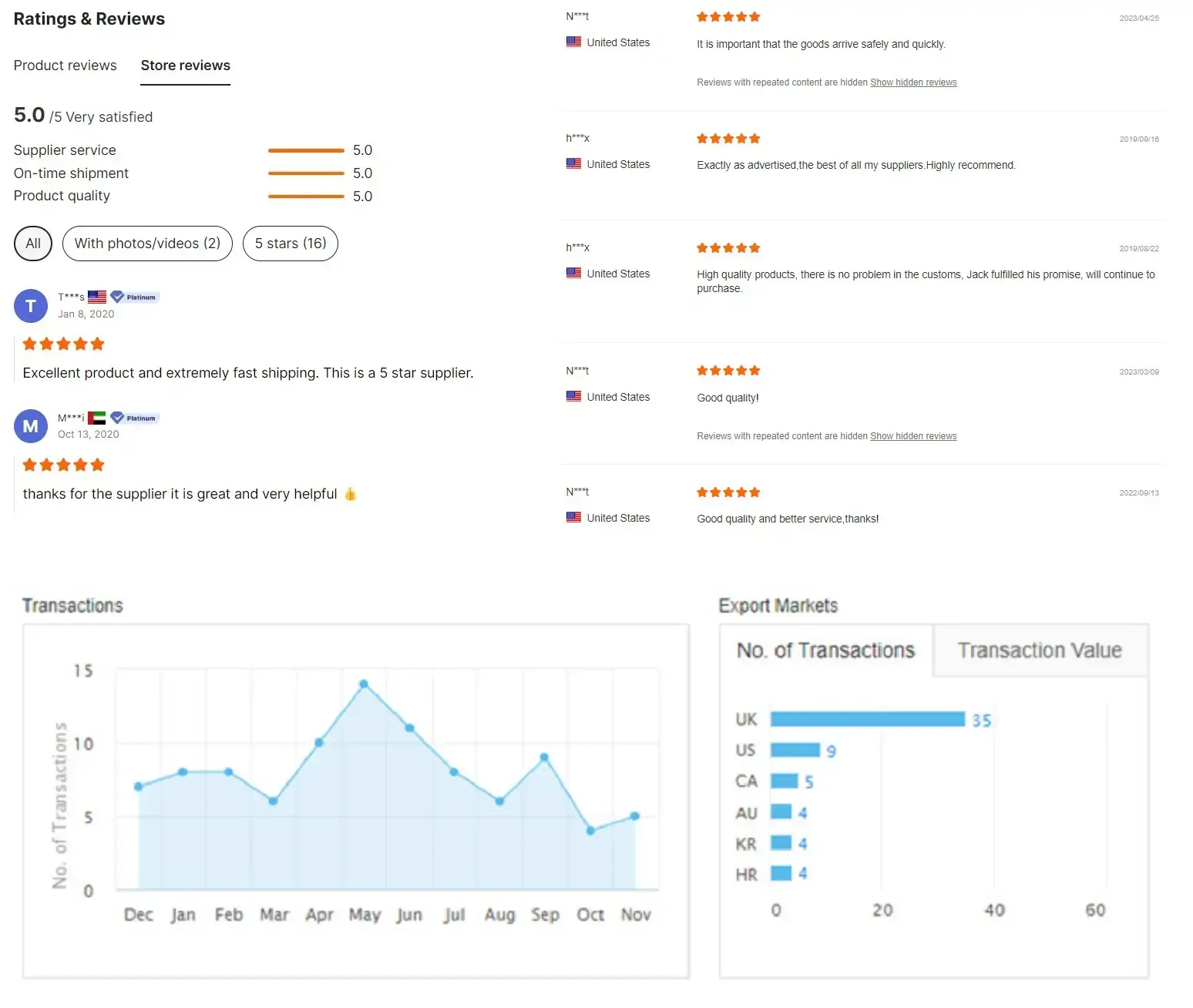
Customer feedback
Xi'an Yihui certificates

Welcome to Xi'an Yihui Factory

Our Advantage
Rich experience: we have 13 Years of professional experience;
Customers all over the world: sell to more than 100 countries;
Provide diversified products: the products have been applied to all major international brands in the fields of drugs, dietary supplements, cosmetics, animal nutrition and functional food.
Price advance: low MOQ with competitive price;
Quality certification: ISO; Halal; Kosher certified
After-sales service: Professional team 7*24 hours customer service.
Contact Us:
Yihui Pharmaceuticals proudly stands as a professional manufacturer and supplier of Veliparib ABT-888. Our commitment to quality is unwavering, as evidenced by our adherence to international standards and the acquisition of ISO, Kosher, Halal, and GMP certifications. For inquiries or to purchase ABT-888, please contact us immediately at sales@yihuipharm.com.
In conclusion, Veliparib ABT-888 by Yihui Pharmaceuticals is not just a pharmaceutical product; it represents a breakthrough in cancer treatment with widespread applications across various industries. Our dedication to excellence and adherence to international standards make us the preferred choice for professionals in the pharmaceutical and research sectors.
Send Message
If you have any enquiry about quotation or cooperation, please feel free to email us at E-mailor use the following enquiry form. Our sales representative will contact you within 24 hours.Thank you for your interest in our products.

.webp)

.webp)
.webp)






