Ticagrelor Api
CAS No.: 274693-27-5
MOQ: 100g
Assay: 99%
Brand: YIHUI
Packing: 100g;1kg;10kg
Supply Ability: 200kg per month
Used: medicine grade; or research
Delivery Time:in stock
Shelf Life: Two years
Can’t sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
What is Ticagrelor api?
Ticagrelor API 274693-27-5 is a brand-new class of antiplatelet aggregation drug created by AstraZeneca in the US. It's the first reversible oral P2Y12 adenosine diphosphate receptor antagonist ever created. The drug has a considerable inhibitory impact on platelet aggregation brought on by adenosine diphosphate( ADP), and it can reversibly act on the P2Y12 subtype of purinoceptor 2 receptors on vascular smooth muscle cells( VSMCs) without taking metabolic activation. also, it has an immediate effect after oral administration and can significantly lessen symptoms in people with acute coronary pattern. In discrepancy to thienopyridine specifics, Ticagrelor API is a reversible P2Y12 receptor asset, making it especially useful for individualities who need anticoagulant remedy previous to surgery.
AstraZeneca started developing ticagrelor in 1999. In 2009, the European Society of Cardiology( ESC) released the results of its Phase III trial, detailing its efficacity in treating cases with acute coronary pattern( ACS). In November 2009, AstraZeneca submitted a new medicine operation for ticagrelor to the European Union and theU.S. Food and Drug Administration( FDA).
In December 2010, ticagrelor entered blessing from the European Union for the forestallment of atherosclerotic thrombotic events in adult cases with ACS.
still, on December 17, 2010, the FDA decided to delay the blessing of AstraZeneca's new antiplatelet medicine ticagrelor and requested fresh analysis of the PLATO study on platelet inhibition and case prognostic.
In January 2011, ticagrelor was officially retailed in all EU member countries under the brand name Brilique, with a lozenge of 90 mg per tablet and a package size of 60 tablets.
On July 20, 2011, AstraZeneca blazoned that the FDA had approved ticagrelor for the reduction of cardiovascular death and heart attack in cases with ACS.
Ticagrelor has been approved for marketing in 41 countries to date and has been included in the medical payment compass in seven countries, similar as the UK.
Basic Information
Product name: Ticagrelor
CAS:274693-27-5
MF: C23H28F2N6O4S
MW:522.57
EINECS:619-540-9
MDL No.:MFCD09954148
Melting point:138-140 ℃
Boiling point:777.6±70.0 ℃(Predicted)
Density 1.67
storage temp. Keep in dark place,Inert atmosphere,Store in freezer, under -20°C
Stability: Stable
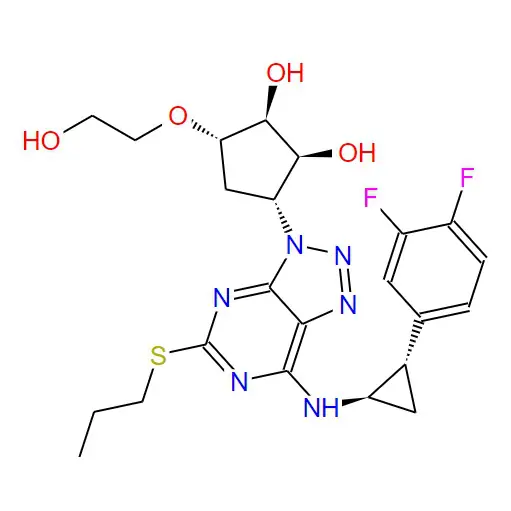
Structural formula:
Quality standard
Test item | Specification | Result |
【Characters】 | ||
Appearance | White or off-white solid | Off-white solid |
Solubility | Freely soluble in Methanol | Conform |
【Identification】 | ||
HPLC | The principal peak in the chromatogram obtained with the test | Conform |
【Tests】 | ||
Moisture | ≤0.50% | 0.22% |
Related substances | Purity≥99.00% | 99.7% |
Maximum single impurity≤0.10% | 0.03% | N.D. |
Residue on ignition | ≤0.2% | 0.03% |
CONCLUSION | THE QUALITY OF THE ABOVE-MENTIONED GOODS IS IN CONFORMITY WITH THE | |
Function
Ticagrelor api is an antiplatelet aggregation drug primarily used for the forestallment and treatment of cardiovascular conditions. Its main functions include
Inhibition of platelet aggregation it can bind to the P2Y12 receptors on platelets, limiting their activation by ADP and dwindling platelet aggregation and clot conformation, hence precluding thrombosis.
Cardioprotection it has a direct cardioprotective effect in addition to its antiplatelet conduct. Myocardial ischemia and injury are dropped, coronary roadway blood inflow is increased, and myocardial oxygen force is bettered.
Treatment of acute coronary pattern( ACS) it's constantly used in the treatment of acute coronary pattern( ACS). It can be used to lower the trouble of cardiovascular events in cases with stable- ST-member elevation myocardial infarction( NSTEMI), and ST-member elevation myocardial infarction( STEMI).
In conclusion, Ticagrelor intermediate can help thrombosis, lower the threat of cardiovascular events, and play a significant part in the treatment of ACS due to its repression of platelet aggregation and direct cardioprotective goods. Please be aware that it's pivotal to follow a healthcare professional's advice and completely read the applicable medicine instructions before taking any drug.
Application
The following places are where ticagrelor is most commonly used:
Treatment of Acute Coronary Syndrome( ACS) Stable- ST- member elevation myocardial infarction( NSTEMI), and ST- member elevation myocardial infarction( STEMI) are all included in the treatment of acute coronary pattern( ACS). To lower the trouble of myocardial infarction, stroke, or mortality in ACS cases, ticagrelor is administered as an antiplatelet treatment.
Post-coronary roadway stenting treatment it's constantly used with aspirin for individuals who have had coronary roadway stenting. This lessens the liability of cardiovascular events brought on by arterial narrowing and helps help thrombosis and restenosis.
Long-term treatment of high-threat cardiovascular cases Cases with high cardiovascular threat who need long-term care can profit from ticagrelor. These cases may have diabetes or have multitudinous fresh cardiovascular threat factors. It may reduce the chance of passing cardiovascular events including heart attacks and strokes.
Please be apprehensive that Ticagrelor should only be used under the supervision of a croaker, and that side goods and any medicine relations should be precisely considered. It's stylish to speak with a healthcare professional for technical guidance in any given case.
Packing & Shipping
Packing: 1kg/foil bag;5kg/carton;25kg/fiber drum; or packing as your request.
Customization: Customized logo; Customized packaging; Graphic customization
Shipping:
Item | Quantity | ETA time | Shipping Method |
By Courier | ≤50kg | 7-15days | Fedex, DHL,UPS,TNT,EMS ect. Fast and convenient |
By Air | 50kg~200kg | 3-5days | Fast and cheap |
By Sea | Large quantity | 20-35days | Cheapest way |
Payment Term
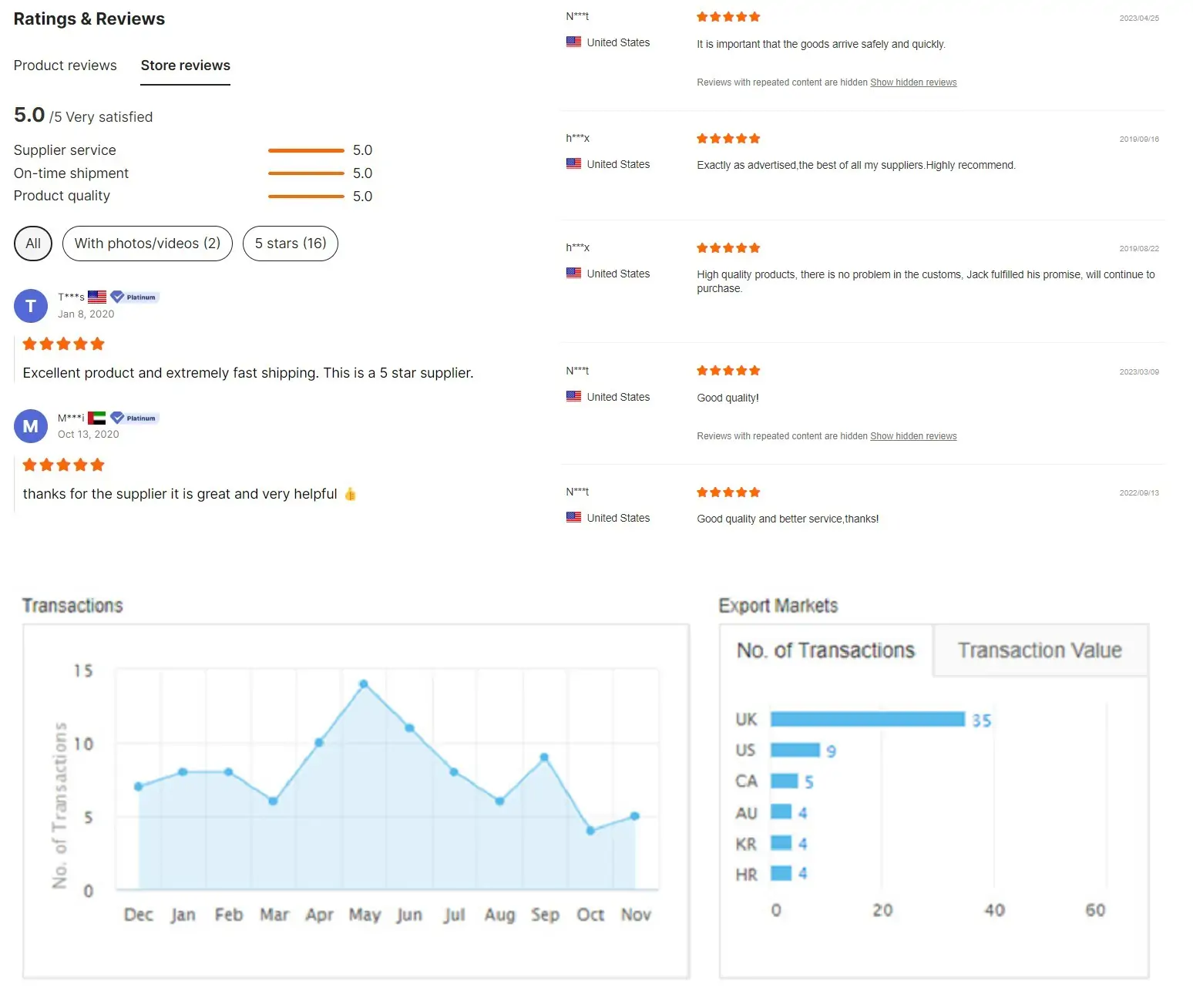
Why Choose Xi'an Yihui?
Customer feedback
Xi'an Yihui certificates
Xi'an Yihui Factory & Warehouse
Our Advantage
Rich experience: we have 13 Years of professional experience;
Customers all over the world: sell to more than 100 countries;
Provide diversified products: the products have been applied to all major international brands in the fields of drugs, dietary supplements, cosmetics, animal nutrition and functional food.
Price advance: low MOQ with competitive price;
Quality certification: ISO; Halal; Kosher certified
After-sales service: Professional team 7*24 hours customer service.
In conclusion
In summary, as a professional Ticagrelor api manufacturer, we have advanced technology, scale advantage, the best quality, rich production experience, and excellent services. These advantages will make it stand out in the market competition and win more customer trust and support. if you need Ticagrelor, pls feel free to contact us any time. we will reply you asap.
our contact information:
E-mail: sales@yihuipharm.com
Tel: 0086-29-89695240
WeChat or WhatsApp: 0086-17792415937
Hot Tags: Ticagrelor api, 274693-27-5, Ticagrelor intermediate,Suppliers, Manufacturers, Factory, Bulk, Price, Wholesale, In Stock, Free Sample, Pure, Natural
Send Message
If you have any enquiry about quotation or cooperation, please feel free to email us at E-mailor use the following enquiry form. Our sales representative will contact you within 24 hours.Thank you for your interest in our products.