Cabazitaxel API
Appearance: White to Off-White powder
Standard: in-house
Application: Anti-Cancer & lab research
Brand: YIHUI
Packing: 1g;10g;100g
Supply Ability: 20kg per month
Shelf Life: Two years
Payment: T/T, LC or DA
Delivery Time: Ready Stock in Local Warehouse,1-3 days
Can't sell to individuals
- Fast Delievery
- Quality Assurance
- 24/7 Customer Service
Product Introduction
What is Cabazitaxel?
Cabazitaxel API is a chemotherapy medication used to treat advanced prostate cancer that has spread to other parts of the body, such as the bones. It belongs to a class of drugs called taxanes, which work by interfering with the ability of cancer cells to divide and grow.
Basic Information
Product Name: Cabazitaxel
CAS: 183133-96-2
MF:C45H57NO14
MW:835.93
EINECS:680-632-7
MDL No.:MFCD18827611
Purity: 99%
Package: 10g;100g;1KG ;5kg
Used: Pharma grade &Lab research
Stability: Stable
Structural formula:
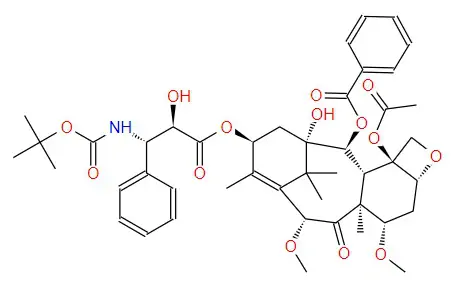
Specification Parameter:
Test Item | Standard | Test Result | |
Appearance | White or almost white powder | White powder | |
Identification (HPLC) | The retention time of the major peak in the chromatogram of the assay preparation corresponds to that in the chromatogram of the standard, as obtained in the assay. | Conform | |
Identification(IR) | The Infra-red absorption spectrum in potassium bromide dispersion of simple exhibits maximal at the same wave length as per that of similar preparation of paclitaxel working standard | Conform | |
Clarity and color of solution | Clear and colorless | Conform | |
Water | ≤1.00% | 0.8% | |
Loss on drying | ≤1.00% | 0.4% | |
Specific rotation | -39.0°~-45.0° | -42.5° | |
Heavy Metals | ≤20ppm | Conform | |
Residual Solvents | Methanol | ≤0.3% | 0.005% |
Dichloromethane | ≤0.06% | 0.007% | |
N-Hexane | ≤0.029% | 0.001% | |
Acetic Ether | ≤0.5% | Not Detected | |
Tetrahydrofuran | ≤0.072% | 0.003% | |
Cyclohexane | ≤0.388% | 0.001% | |
Unknown single impurity | ≤0.20% | 0.04% | |
Total impurities | ≤1.00% | 0.08% | |
Purity (HPLC) | ≥99.00% | 99.92% | |
Conclusion: Comform | |||
Chemical Composition:
Explore the precise chemical makeup of it in the table below:
| Component | Percentage Composition |
|---|---|
| Cabazitaxel | 98% |
| Excipients | 2% |
Effects and Functions:
Cabazitaxel works as a strong anticancer specialist, essentially utilized in the therapy of metastatic maiming safe prostate disease (mCRPC). Its system of activity includes upsetting microtubule elements, hindering cell division, and prompting apoptosis in disease cells. The product produced by Yihui represents a significant development in cancer treatment, providing patients with hope and improved outcomes.
Synthesis Process:
The synthesis of Cabazitaxel API involves a meticulous process ensuring purity and efficacy. Starting from 10-deacetylbaccatin III, Yihui employs state-of-the-art technologies to introduce semi-synthetic modifications, resulting in the production of it with a purity exceeding 98%. This process underscores Yihui's commitment to pharmaceutical innovation and quality assurance.
Quality Standards:
Yihui sticks to rigid quality norms to ensure the viability and security of 183133-96-2. Our assembling processes line up with worldwide benchmarks, and we gladly hold ISO, Genuine, Halal, and GMP accreditations. This obligation to quality guarantees that each bunch of the item fulfills or surpasses industry guidelines, imparting trust in our clients and end-clients.The quality guidelines of the product can be portrayed as follows:
Substance Immaculateness: It should fulfill severe synthetic virtue guidelines to guarantee that it contains no pollutants that might be destructive to patients or influence its remedial viability. The immaculateness of it is regularly estimated utilizing superior execution fluid chromatography (HPLC) or other insightful techniques.
Actual Properties: It should likewise fulfill explicit actual property guidelines, including molecule size, liquefying point, and solvency. These properties are pivotal to the medication's plan and similarity with other excipients or conveyance frameworks.
Solidness: The product should be steady under many circumstances, including light, temperature, and moistness. Dependability testing is led to guarantee that the medication holds its quality and adequacy all through its time span of usability.
Lingering Solvents: The Product should meet severe cutoff points on remaining dissolvable substance to guarantee patient wellbeing. The leftover dissolvable substance is commonly estimated utilizing gas chromatography (GC) or other logical strategies.
Microbiological Defilement: Cabazitaxel API should be liberated from microbiological pollution, including bacterial and contagious development. This is especially significant for injectable plans, where microbial pollution can actually hurt patients.
Weighty Metals: It should meet severe cutoff points on weighty metal substance, including lead, mercury, and arsenic. These metals can be destructive to patients and may influence the medication's adequacy.
Administrative Consistence: It should consent to all applicable administrative necessities, including Great Assembling Practice (GMP) guidelines and pharmacopoeial principles. These guidelines are set up to guarantee that the product is created reliably and satisfies the best guidelines.
Application Fields in Various Industries:
The product's flexibility stretches out past the drug domain. Its application traverses assorted enterprises, including research organizations, biotechnology organizations, and drug producers. Yihui's item finds utility in spearheading research, drug improvement, and the union of novel drug compounds, making it a significant resource chasing clinical progressions.
OEM Services:
As well as offering Cabazitaxel API, Yihui gives Unique Gear Assembling (OEM) administrations. Team up with us to alter details, bundling, and marking to meet your particular prerequisites. A seamless partnership for the production of pharmaceuticals of high quality is made possible by Yihui's dedication to adaptability and client satisfaction.
Contact Us:
Yihui, a distinguished Cabazitaxel manufacturer and supplier, prioritizes product quality and compliance with international standards. With ISO, Kosher, Halal, and GMP certifications, we assure our clients of pharmaceutical excellence. For inquiries or to place an order, contact us at sales@yihuipharm.com. Yihui invites you to embark on a journey of innovation and reliability in pharmaceutical manufacturing.
Send Message
If you have any enquiry about quotation or cooperation, please feel free to email us at E-mailor use the following enquiry form. Our sales representative will contact you within 24 hours.Thank you for your interest in our products.











